
Features
From exclusive tours inside top organisations, to interviews with industry experts and opinion pieces written by quality innovators, read the latest features to improve your knowledge and understanding.

Arming the quality professional
Should quality professionals be seen only as the enforcers of quality? Paul Vaughan CQP FCQI outlines the key role of open communication to avoid problems before they occur.

What are we going to do with artificial intelligence?
Artificial intelligence is integral in many sectors, and quality management systems are no exception, says senior auditor Pedro Mejias.

AI automation driving digital acceleration in testing
Mav Turner, CTO of DevOps at Tricentis, takes a closer look at the use of AI in quality assurance to drive software development.

Building a quality culture
A quality culture is at the heart of any successful organisation. Consultant and communication coach Lesley Worthington outlines the key building blocks to achieve it.

Engagement of people
Raffat Khatoon Mohammed, CQP MCQI, ISO 9001, 14001 and 45001 Lead Auditor at Qatar Primary Materials Company (QPMC), explains how ‘engagement of people’ helped QPMC to overcome staff and skill shortages during the Covid-19 pandemic.
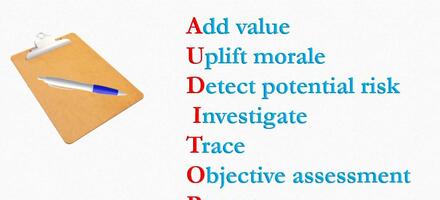
The future value of auditing from an industry perspective
Cheekeong Loh, CQP MCQI, MIEAust CPEng, outlines how auditing can develop in the future.

Eliminating the costs of poor quality
Vimala Balusamy, CQP MCQI, Quality and Project Management Consultant, India, shares her five steps to help prevent poor quality costs in businesses.

Strengthening customer relationships through quality assurance
Ben Dewison, Specialist Member of The Institute of Customer Service, UK, explains the importance of quality assurance and how it can be used to improve customer relationships.

Setting and applying SMART objectives
Sam Kinch, Quality Management Consultant at qualityinspired.co.uk, highlights the importance of SMART objectives and how businesses should apply them.

Error-proofing production for medical devices
Anca Thompson, Senior Vice President and Chief Quality Officer at Sanmina Corporation, San Jose, California, explores how medical device manufacturers can error-proof their production process using technology and automation.
Recommend membership to a colleague and get £25*
1. Fill in a simple form
2. We’ll send them an invite
3. They join CQI or IRCA
4. You get rewarded
Interested in training?
Take this quick quiz and find the training that’s right for you.