
Technical resources
Read the latest on the ISO 14001:2015 certification process, how to conduct an internal audit, how to comply with ISO 9001:2015, and more.

Take a risk to mitigate climate change
Could a structured risk assessment help organisations achieve the UN’s Sustainable Development Goals while mitigating climate change, asks Zoi Kontodimou, Chair of the CQI’s Sustainability special interest group.

Auditing climate change
Richard Green, founder and owner of Kingsford Consultancy Services, examines the implication of ISO’s climate change amendments to management system standards for auditors.

Taking action on climate change
In February, the International Standards Organization (ISO) added two new statements of text into 31 Type A management system standards, to ensure that climate change issues are considered. Geoff Vorley, Director of Quality Management and Training Limited, UK, takes a closer look at the impact of this on several of the key standards.

How QMSs can ensure safety in medical devices
Karandeep Singh Badwal, Director of QRA Medical and an internal auditor for ISO 13485, outlines some of the common mistakes found in the quality management systems for medical devices.

Conformity assessment: Setting the standard
In this interview, Warren Merkel, Vice President of Policy at the ANSI National Accreditation Board, explains the reasons behind the update to ISO/IEC 1706:2022 Conformity assessment – Code of good practice, and its impact on the work of quality professionals.

ISO 9004:2018 – little known but highly useful
Carew Hatherley, Managing Director of IT consulting company IQM Group, examines how the scoring system of ISO 9004 can be used for a different style of reporting or analysis.

Preventing the recurrence of a nonconformance
Ravindiran Gurusamy, IRCA Certificated Associate Auditor, offers an overview of nonconformance and the corrective action process for management system standards.
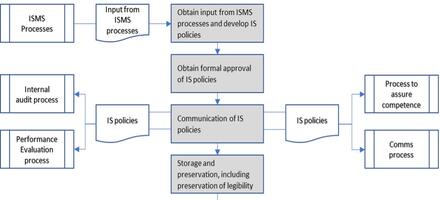
ISO/IEC TS 27022:2021 – Information technology – Guidance on information security management system processes
Richard Green, CQP FCQI, Managing Director of Kingsford Consultancy Services, UK, outlines the newest guidance on information security implementation and operation.

The High Level Structure is dead. Long life to the Harmonised Approach?
Horacio Martirena, a CQI technical assessor, IRCA Lead Auditor and Independent Consultant in management systems, outlines the development of the Harmonised Approach and the issues it addresses, and what it needs to resolve.

Preventing infectious disease hazards with ISO 45006
Richard Green provides an overview of the draft version of ISO 45006. The new Occupational health and safety management standard, that will provide organisations with guidelines for preventing and managing infectious disease hazards.
Interested in training?
Take this quick quiz and find the training that’s right for you.