Search
Search our content by date or relevance.

Quality assurance in the supply chain
At global supply chain service group Czarnikow, CEO Robin Cave says a philosophy of continuous improvement leads to consistency of quality.

The continuing relevance of ISO 9004
ISO 9004 should be seen as more than pure guidance if it is to help ensure standards for today’s organisations, says Richard Green, Senior Consultant at Kingsford Consultancy Services.

Upgrading standards for the global moving business
Due to the impact of the global pandemic and ever-increasing scrutiny of the environmental practices of relocation businesses, the industry is gearing up for an important update to its quality standard.

A joined-up approach to building safety
The UK is introducing the most significant reform to building safety regulations in decades, aiming to create a coherent system that defines responsibilities and improves quality across the built environment.

The role of quality assurance in the UK energy sector transformation
For Ivan Ericsson, Head of Quality Management at engineering and management consultants Expleo UK, treating assurance as a strategic tool, rather than just a box-ticking exercise, is critical to the future of the UK energy sector.

Engagement of people
Raffat Khatoon Mohammed, CQP MCQI, ISO 9001, 14001 and 45001 Lead Auditor at Qatar Primary Materials Company (QPMC), explains how ‘engagement of people’ helped QPMC to overcome staff and skill shortages during the Covid-19 pandemic.

The High Level Structure is dead. Long life to the Harmonised Approach?
Horacio Martirena, a CQI technical assessor, IRCA Lead Auditor and Independent Consultant in management systems, outlines the development of the Harmonised Approach and the issues it addresses, and what it needs to resolve.

Resilience at the heart of the nation’s defence
Lisa Taylor, CQP MCQI, Corporate Quality Manager at Dstl, explains how the organisation's resilience planning meant it was able to pivot quickly to remote working and continue to support government and its existing customers in the UK’s response to Covid-19.
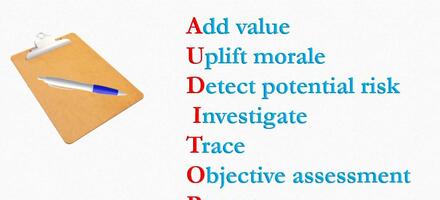
The future value of auditing from an industry perspective
Cheekeong Loh, CQP MCQI, MIEAust CPEng, outlines how auditing can develop in the future.

Managing supply chains post Brexit
Malcolm Harrison, Group CEO of the Chartered Institute of Procurement & Supply (CIPS), talks to Daniel Moore about how Brexit will impact supply chains and logistics in 2021.