Search
Search our content by date or relevance.

Engagement of people
Raffat Khatoon Mohammed, CQP MCQI, ISO 9001, 14001 and 45001 Lead Auditor at Qatar Primary Materials Company (QPMC), explains how ‘engagement of people’ helped QPMC to overcome staff and skill shortages during the Covid-19 pandemic.

Serious skills shortage in quality management: Act now
For some time now, quality leaders have been worried about the growing skills deficit in our profession. So when the CQI and our corporate partners got together at a recent event, everyone agreed on the need for action.
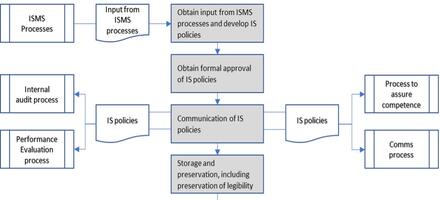
ISO/IEC TS 27022:2021 – Information technology – Guidance on information security management system processes
Richard Green, CQP FCQI, Managing Director of Kingsford Consultancy Services, UK, outlines the newest guidance on information security implementation and operation.

Bouncing forward in the wheel of life: how to face disruption and create balance
Eva Provedel, Leadership Coach and Trainer at Go Mind in Motion Limited, and author of two recent books on resilience, explains the importance of a positive mindset and balance on quality of life.

Applying Appreciative Inquiry in ISO 9001 Quality Management Systems Auditing
Jessen Yeoh, CQP, MCQI, IRCA QMS/EMS/OHS Principal Auditor, Principal Advisor, P Excel Advisory Pty Ltd, explains the benefits of the Appreciative Inquiry approach in auditing.
7 hours
MD-QMS ISO 14971:2019 Foundation (Medical Device – Application of risk management to medical devices)
This course provides a basis for learners who wish to go on to complete CQI and IRCA Certified MD-QMS Auditor Training courses

The High Level Structure is dead. Long life to the Harmonised Approach?
Horacio Martirena, a CQI technical assessor, IRCA Lead Auditor and Independent Consultant in management systems, outlines the development of the Harmonised Approach and the issues it addresses, and what it needs to resolve.

Innovation, standards and the battle for global competitiveness
The UK Quality Infrastructure (UKQI) - an alliance of four institutions which oversees standardisation, testing, measurement, certification and accreditation in the UK, has stepped up its response to Industry 4.0. Why?

Preventing infectious disease hazards with ISO 45006
Richard Green provides an overview of the draft version of ISO 45006. The new Occupational health and safety management standard, that will provide organisations with guidelines for preventing and managing infectious disease hazards.
The future value of auditing from an industry perspective
Cheekeong Loh, CQP MCQI, MIEAust CPEng, outlines how auditing can develop in the future.